An expert panel’s 6-year-old recommendations on RHD genotyping are not quite being used as intended. Let’s learn how we can do better!
NOTE: Continuing Education credit for this episode has expired. See below for details.

Sue Johnson MSTM, MT(ASCP)SBB
Sue is Back!
In this interview, my dear friend Sue Johnson, one of the authors of the updated recommendations published in 2020, will address challenges laboratories and clinicians have faced in implementing the 2015 recommendations, outline the potential advantages to RHD genotyping, and summarize the updated recommendations.
This interview was recorded LIVE at an educational seminar jointly sponsored by the California Blood Bank Society and LifeStream Blood Bank. It is presented with minimal edits so you can experience it the way it happened. My thanks to both organizations for permission to use the recording.

Sue Johnson MSTM, MT(ASCP)SBB
Sue is Back!
In this interview, my dear friend Sue Johnson, one of the authors of the updated recommendations published in 2020, will address challenges laboratories and clinicians have faced in implementing the 2015 recommendations, outline the potential advantages to RHD genotyping, and summarize the updated recommendations.
This interview was recorded LIVE at an educational seminar jointly sponsored by the California Blood Bank Society and LifeStream Blood Bank. It is presented with minimal edits so you can experience it the way it happened. My thanks to both organizations for permission to use the recording.
About My Guest:
Sue Johnson, MSTM, MT(ASCP)SBBCM is the Director of Clinical Education at Versiti | Blood Center of Wisconsin. She also is the director of the Specialist in Blood Banking Program at Versiti and the Transfusion Medicine Program at Marquette University. Sue is Associate Director of the Indian Immunohematology Initiative, a program designed to improve general immunohematology knowledge in South Asia. She is a sought-after speaker and world-class immunohematology expert.
Continuing Education Expired
This podcast episode offered continuing education credit for two years from its release date, but is no longer eligible for such credit.
To find Blood Bank Guy Essentials Podcast episodes with active continuing education opportunities, Click here or visit Transfusion News Continuing Education on Wiley Health Learning.
DISCLAIMER: The opinions expressed on this episode are those of my guest and I alone, and do not reflect those of the organizations with which either of us is affiliated. Neither Sue nor I have any relevant financial disclosures.
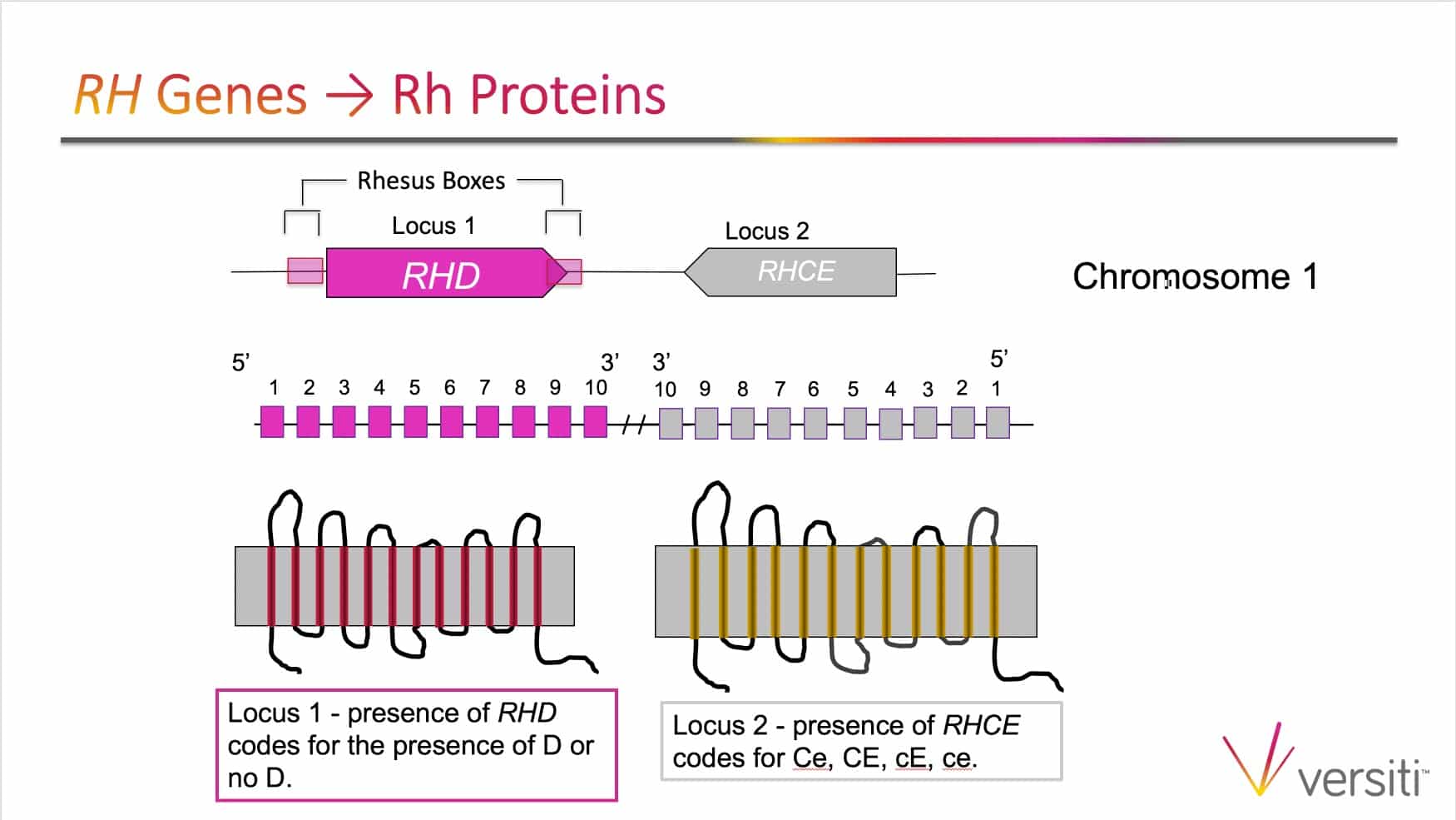
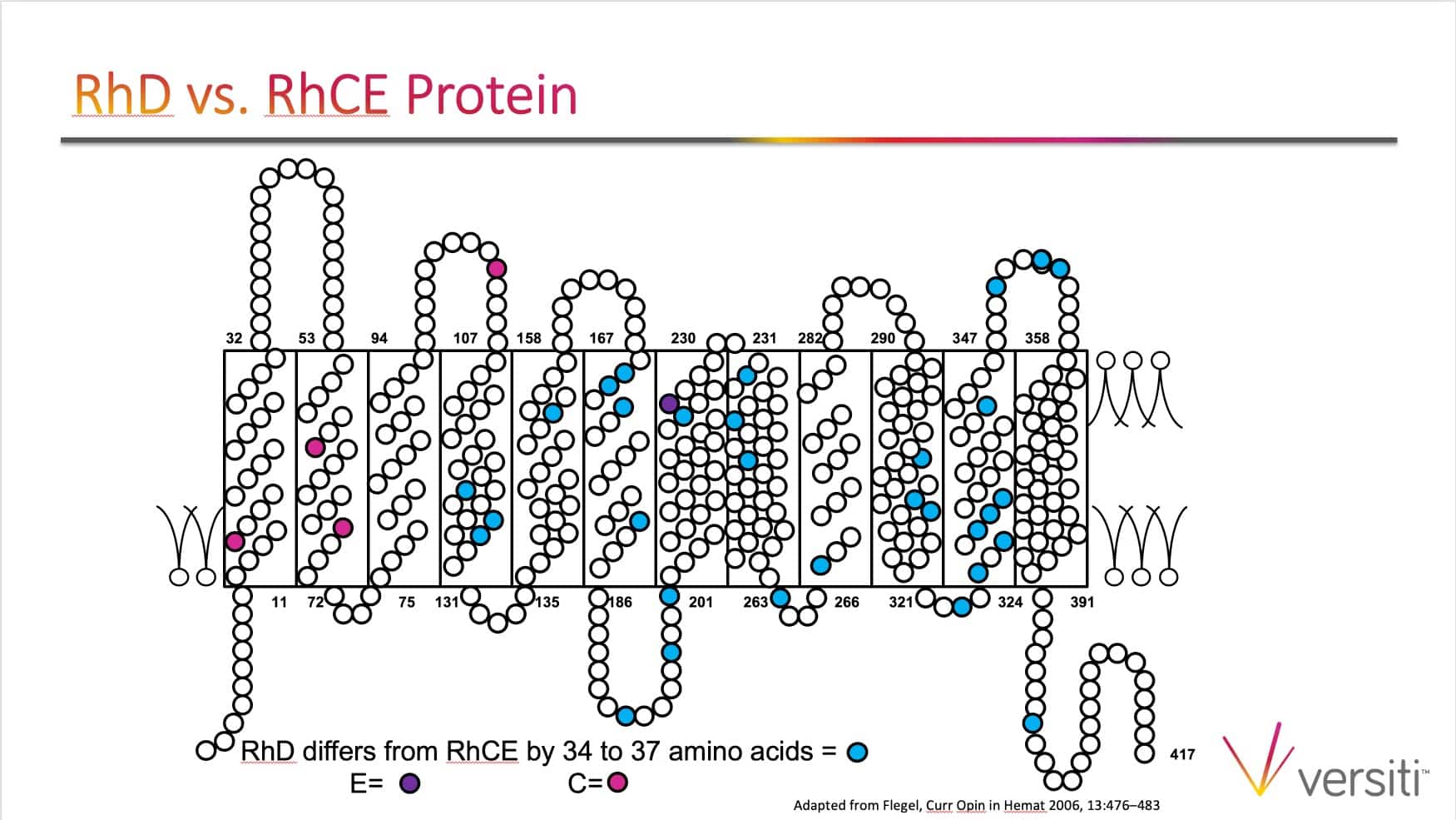
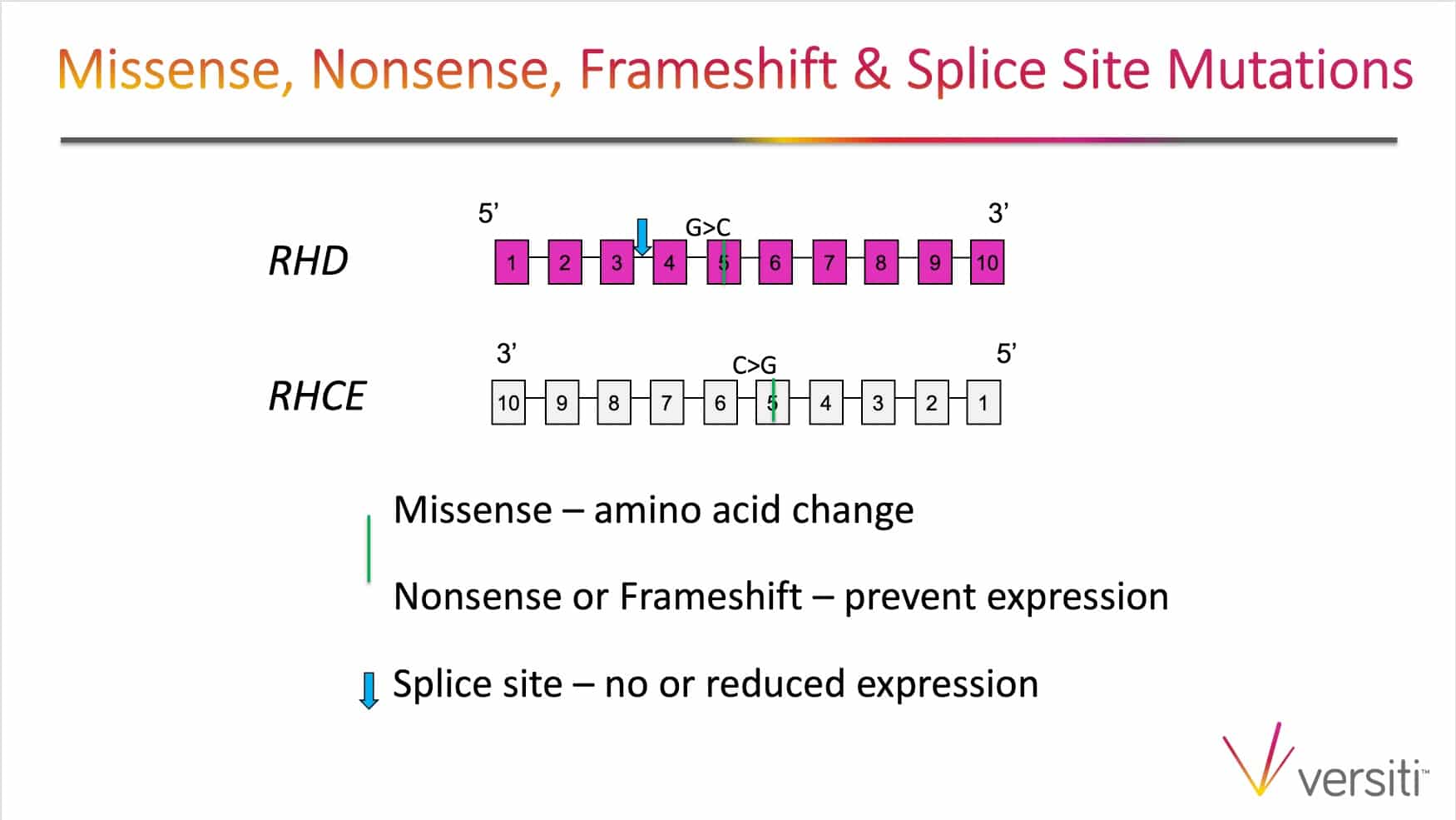
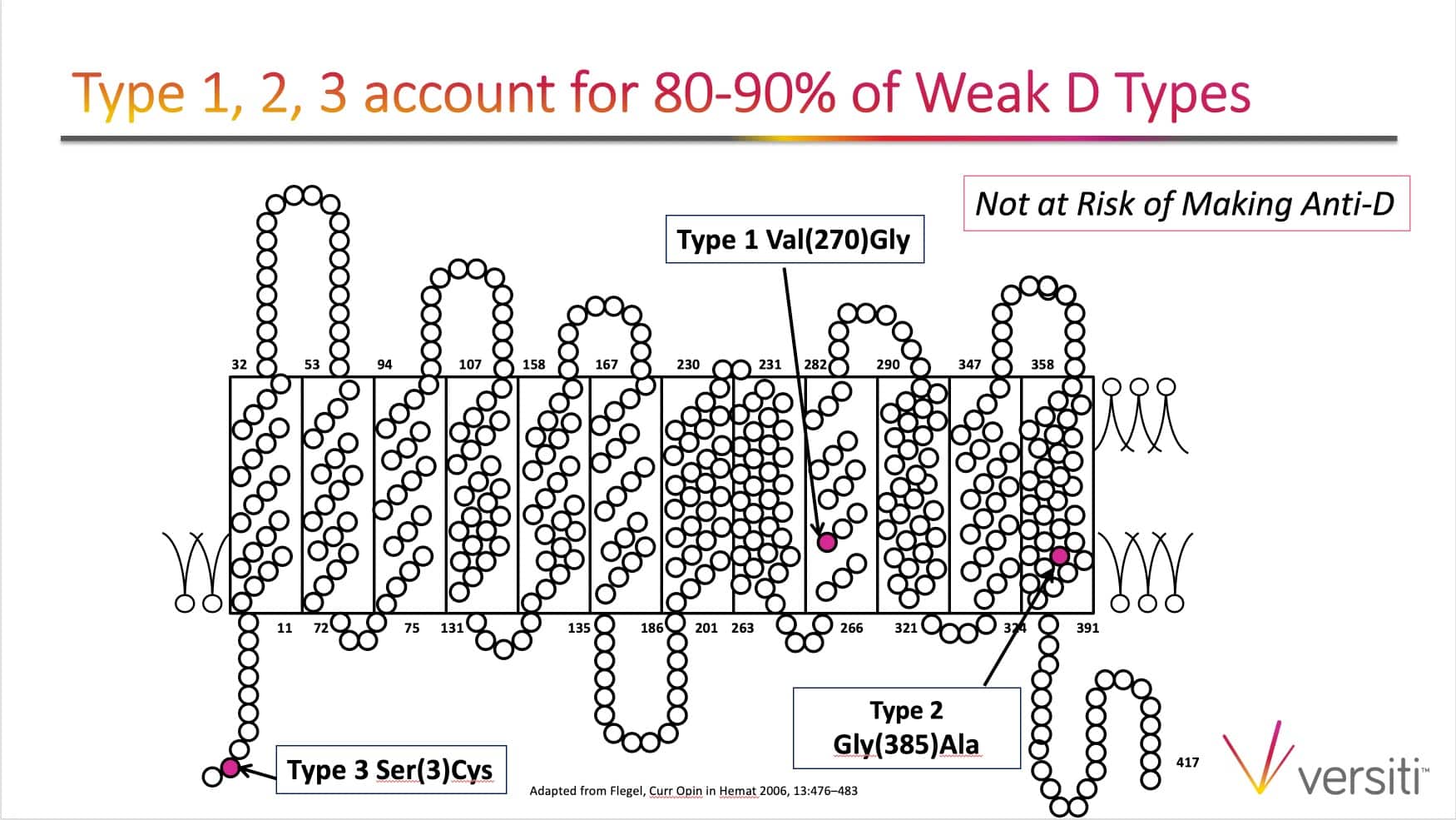
The images below are generously provided by Sue Johnson.
Further Reading:
- Original Working Group Recommendations: Sandler SG et al. It’s time to phase in RHD genotyping for patients with a serologic weak D phenotype. Transfusion 2015;55:680-689
- Updated Recommendations: Flegel WA al. It’s time to phase out “serologic weak D phenotype” and resolve D types with RHD genotyping including weak D type 4. Transfusion 2020;60:855-859
- ACOG Practice Bulletin. Prevention of Rh D Alloimmunization. Number 181, August 2017
Thanks to:
- Samantha Chaffin, Design and content consultant
Music Credit
Music for this episode includes “Cuando te invade el temor” and “Reflejo,” both by Mar Virtual via the Free Music Archive. Click the image below for permissions and license details.

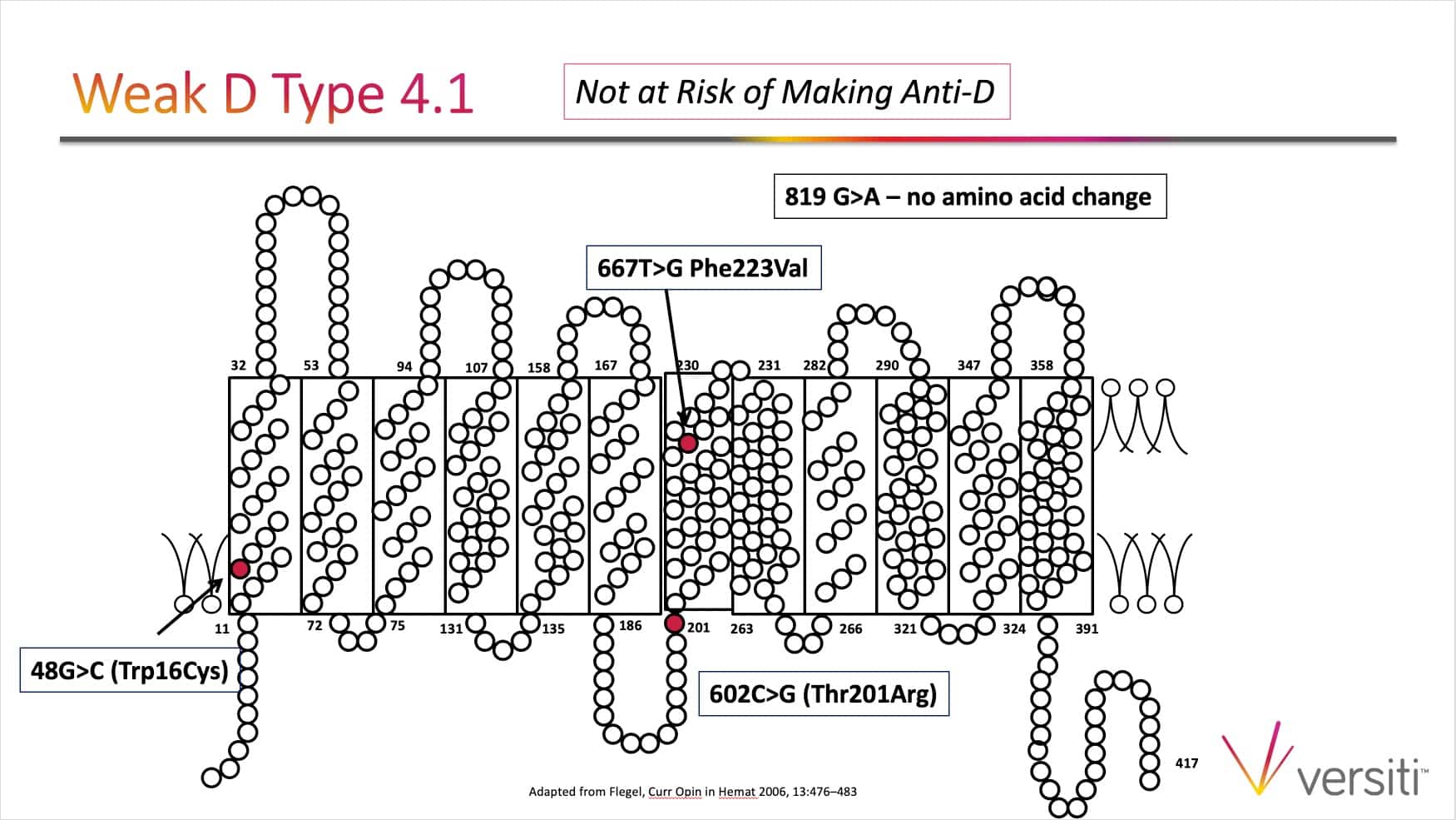
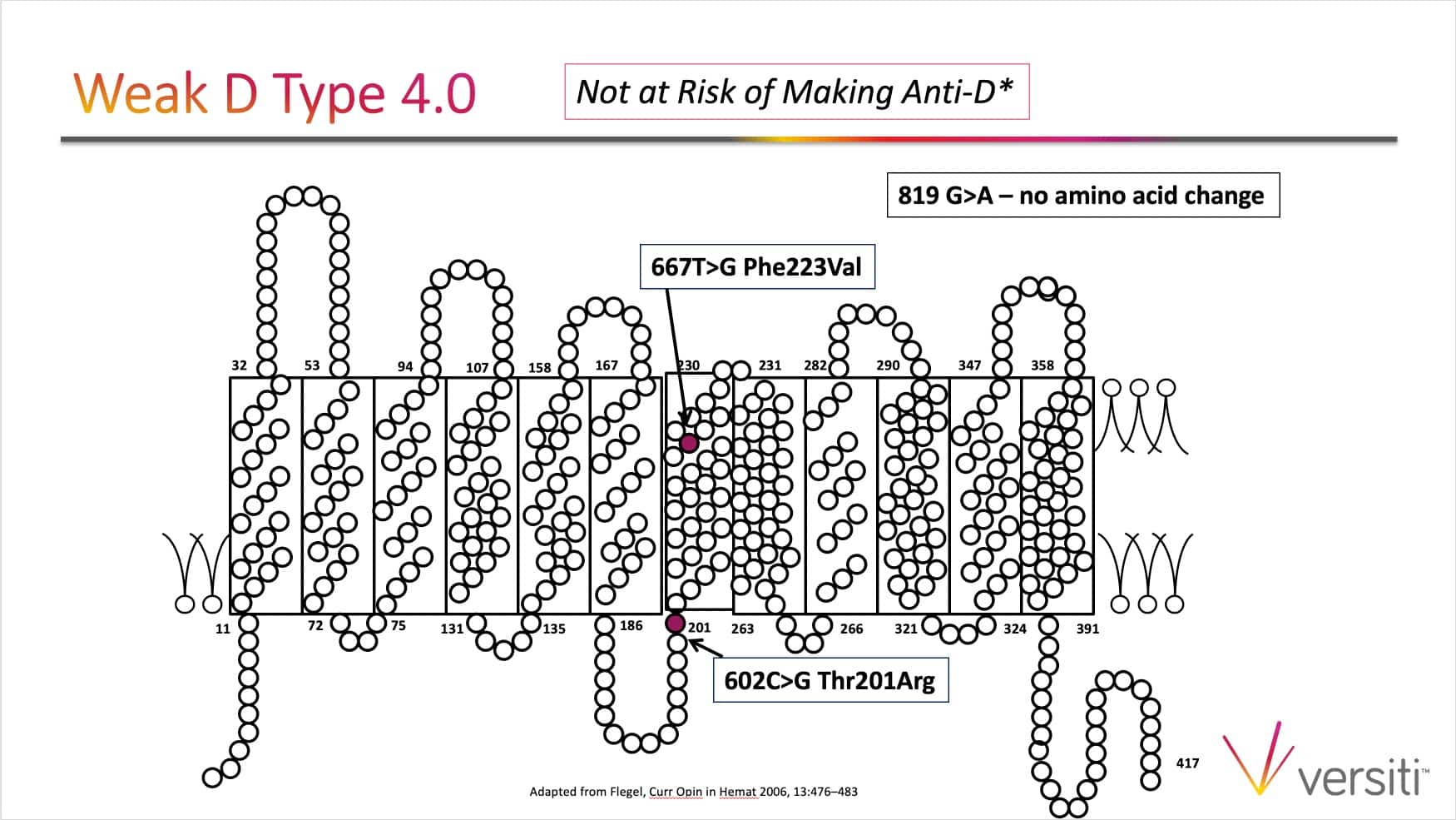









Thanks Sue and Joe for another great podcast. I know of some institutions that perform weak D testing (ie AHG) on all samples, including transfusion recipients (and not just donor samples and cord blood samples), and then follow up with genotyping when the direct (immediate spin typing) is negative but the weak D test (AHG) is positive. I know this policy would result in picking up the DVI variant (which we probably don’t want to detect anyway because they should be treated as D negative from transfusion standpoint). In your opinion is there any added benefit or danger to performing the weak D test (ie AHG) when typing transfusion recipients or prenatal samples (as long genotyping is performed on any patients who are D positive by only the weak D test)? Thanks.
Excellent question Joni! Interestingly, when I started in this amazing field we performed weak D tests on anyone who typed D-negative at IS. However, we stopped doing weak D tests routinely in pretransfusion testing as it created so much confusion on what to call these individuals, D positive or D negative. This was before we had genotyping in the dark ages 😉 Of course dropping the weak D IAT/AHG testing also streamlined testing and saved $ in reagents and laboratory scientist time to perform the test.
To answer your question, there is no danger as you are performing genotyping. I see it as a benefit. If you know a patient has a weak D type that you can call RhD-positive you can transfuse RhD-positive blood, saving precious RhD-negative blood. In prenatal testing you are preventing a women from receiving unnecessary Rh immune globulin. It’s all good!
Our hospital uses an automated platform that seems to detect all wkD/D variants we have tested with the vendor’s ABD gel card (I.e. not IgG card). We recently did a method verification for use of anti-D IAT on the instrument to replace tube weak D testing for cord blood. We tested several donor samples from our supplier, and for each sample: IS was negative, IAT anti-D in tube and on instrument was positive, and the D column of the ABD gel card was positive. While I agree that serologic weak D should be phased out (and that molecular is the ideal), I am wondering how I might go about “proving” that this methodology can be used in place of weak D (IAT) testing of cord blood? We do NOT test prenatal samples (though I admit I am curious about that as well).
Just to make sure I understand the question, you would like to use the automated method in place of performing a serologic weak D IAT in test tubes on cord blood. The risk is that not all weak/partial D will be detected by automation. You could also argue that you may not detect all weak/partial D with a given Anti-D reagent.
If you “miss” a weak/partial D in a cord blood you would likely have a super “positive” rosette test that you can trouble shoot with tube testing.
In my experience there is no way to ensure you will be able to serologically detect all D variants with the current reagents and methods available. Your validation may look fine but know there may be cases that slip through for which you are unable to avoid. This is the fun of immunohematology 😉
The only thing I would add to Sue’s comment is that those decisions must be made in conjunction with your local medical and quality authorities. Deciding on platforms and their uses comes with risk either way, as Sue so accurately points out, so your medical director must bear that responsibility and decide what risk is most appropriate for your setting.
-Joe
The article “It’s time to phase out “serologic weak D phenotype” and resolve D types with RHD genotyping including weak D type 4” is locked behind a paywall. Is there any way I could receive a copy without needing to pay?