Bacterially-tainted platelet units are FAR more common than other transfusion infections! Dr. Anne Eder outlines the problem and offers solutions.

Dr. Anne Eder
A couple of notes
This episode was recorded on March 15, 2016, and was as up-to-date as possible on the day of recording. However, the FDA has since released draft guidance documents that could potentially “change the game” in regard to everything we do to prevent septic reactions from platelet transfusion. Please check FDA and AABB sources for current information in the United States.
Dr. Eder is now working for the Food and Drug Administration; this interview occurred before her employment there, and should not be construed in any way to reflect official FDA policy.
BONUS: Early in the interview, Dr. Eder also gave a great summary of where we were with Zika virus safety measures for prevention of this virus entering the U.S. blood supply. For more information, see Episode 15.

Dr. Anne Eder
A couple of notes
This episode was recorded on March 15, 2016, and was as up-to-date as possible on the day of recording. However, the FDA has since released draft guidance documents that could potentially “change the game” in regard to everything we do to prevent septic reactions from platelet transfusion. Please check FDA and AABB sources for current information in the United States.
Dr. Eder is now working for the Food and Drug Administration; this interview occurred before her employment there, and should not be construed in any way to reflect official FDA policy.
BONUS: Early in the interview, Dr. Eder also gave a great summary of where we were with Zika virus safety measures for prevention of this virus entering the U.S. blood supply. For more information, see Episode 15.
DISCLAIMER: The opinions expressed on this episode are those of my guest and I alone, and do not reflect those of the organizations with which either of us is affiliated (currently or when this interview occurred in 2016). Neither Anne nor I have any relevant financial disclosures.
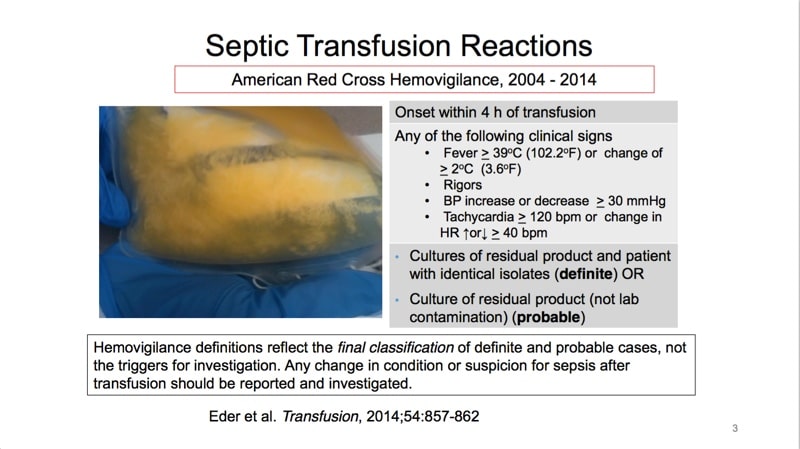
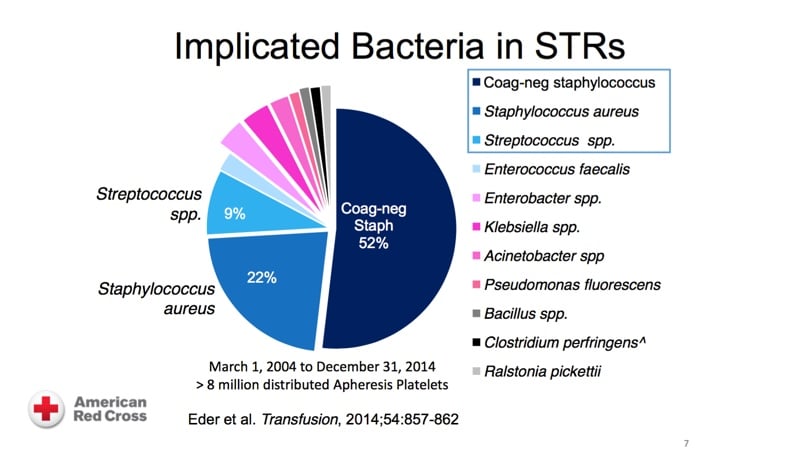
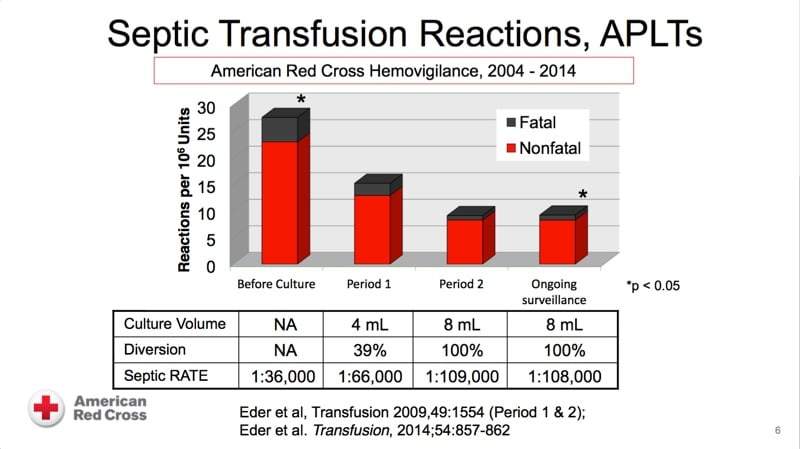
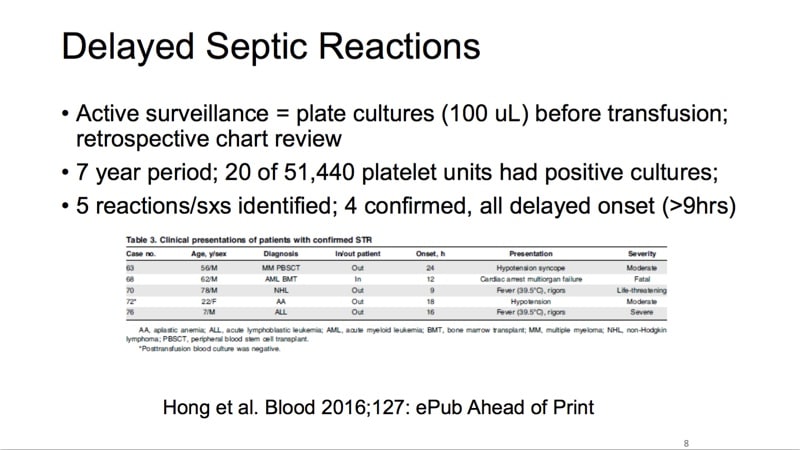
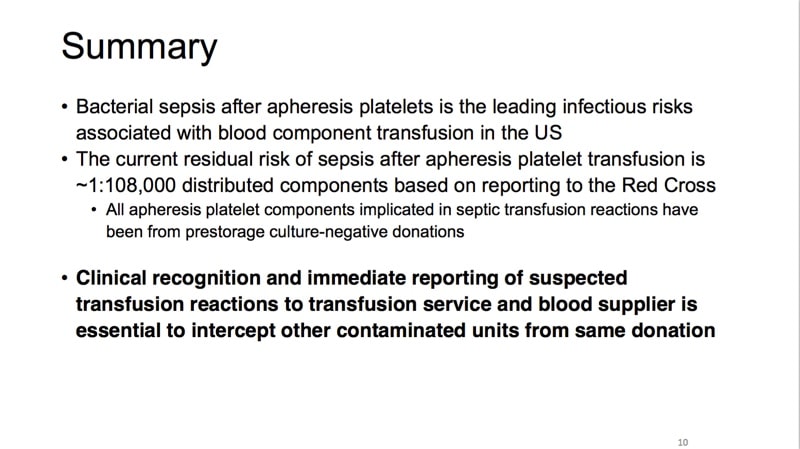
The images below are referenced in Dr. Eder’s discussion. Please click on an image to see a larger version, or click here for the full handout, courtesy of Dr. Eder.










Hello Dr.CHaffin!
So,as far as i understand(correct me if i am wrong!),it is mandatory to do a culture for apheresis platelets 24 hours after they have been collected and if they are not tranfused we should do some kind of rapid test which indicates the presence of bacteria in the 4th day,am i right?
Yannis, in the US, the only current requirement is either bacterial detection testing (typically done at least 24 hours post collection) OR pathogen reduction treatment of the platelet unit. There is currently much discussion here about what to do with products in their 4th and 5th days of storage, but there is currently no requirement to do a rapid test for older products (note that the linked FDA document is a draft and is not binding on anyone at this point).
-Joe
Hi, Dr. Chaffin!
I just discovered your podcast and have been working through all the past episodes. It really is a wealth of helpful information!
I thought you might want to know that the link on the show page for this episode 003 currently actually downloads episode 023 with Jeff Carson instead.
Thanks again for all you do to help those of us who are relatively new to transfusion medicine!
Thanks for letting me know, Jack! It is fixed now. I very much appreciate your kind words about the podcast.
-Joe